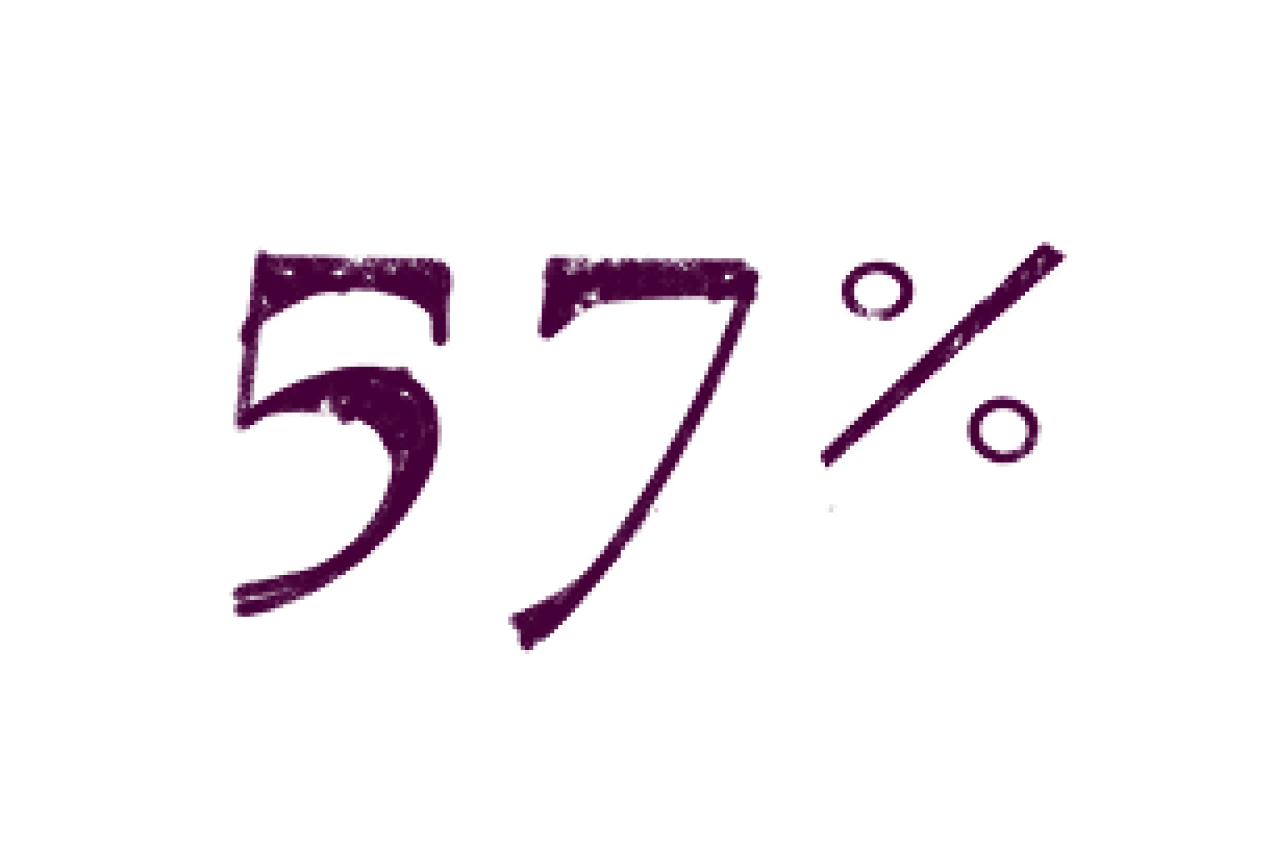The ANCHOR Study
“Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer”
Urgent Need to Screen and Treat Anal Precancerous Conditions
Although anal cancer is still considered rare, cases are rising. A big factor is due to late detection linked to poor survival and treatment causing significant side effects. Unlike cervical cancer, which saw an 80% reduction through screening, no screening guidelines exist for anal cancer.
Populations at Higher Risk for Anal Cancer
- People with immune suppression for solid organ transplants
- Patients who are HIV positive
- Women with a history of vulvar, cervical HSIL, or cervical cancer
Goal of the study:
Anal cancer, like cervical cancer, is caused by HPV 16 and is preceded by high-grade squamous intraepithelial lesions (HSILs), or anal intraepithelial neoplasia (AIN) 2 or 3. The ANCHOR study researches whether treating anal HSILs could similarly reduce progression to anal cancer, as seen with cervical HSIL treatment.
The Study
HIV-positive people, who are more likely to get anal cancer were divided into two groups for study:
- Group 1: Received no treatment but was watched closely
- Group 2: Received treatment for anal HSIL with different methods
Patients were treated until HSIL was gone and had check-ups every six months. If anyone was found to have cancer, they were immediately referred for treatment.
Highly Promising Results
After 25.5 months, the treatment group had 9 anal cancer cases and the monitoring group had 21, indicating a 57% lower incidence in the treatment group.
This suggests that treating precursors may help prevent anal cancer!